Which of the Following Has the Lowest Ionization Energy
First ionization energy is the energy required to separate one valence electron from an atom in gas phase. Hence sulphur has the least ionization enthalpy in this case.
Ionization Energy Trends Of The Periodic Table
Hence sodium electronic configuration 1s22s22p63s1 has the lowest ionisation energy.
. From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Prior to its discovery it was referred to as eka-caesium. So the decreasing order of ionization potential of all four elements is as follows.
Which group has the largest second ionization energy. A Oxygen B Nitrogen C Fluorine D Sulphur. The easier the atom can release the electron the lower the energy required to remove it ie.
From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Sulphur has the maximum atomic radius when compared to the other three atoms. The first ionisation energy is the energy involved in removing one mole of electrons from one mole of atoms in the gaseous state.
Down in a group ionization potential decreases so the ionization potential of sulphur is lower than the oxygen. 119 rows - Ionization energy. Expert answered alvinpnglnn Points 12475.
The group of elements which have. Hence second ionization energy of sodium is largest. Which group has the lowest ionization energy.
Which has lowest first ionization energy. When Na ions loses an electron this stable configuration of Ne is broken. The group of elements which have the lowest ionization energy are the alkali metals.
Hence sulphur has the least ionization enthalpy. Which of the following has the lowest ionization energy. Which element has the lowest ionization level.
Which Of The Following Has The Lowest Ionization Energy. The ionization energy generally decreases from top to bottom in groups due to lower shielding effect and outer electrons are loosely packed so easy to remove and increases from left to right across a period. Thus helium has the largest first ionization energy while francium has one of the lowestThus helium has the largest first ionization energy while franciumfranciumFrancium is a chemical element with the symbol Fr and atomic number 87.
Expert answeredAutaPoints 254 User. Which of the following is the. Which of the following has the lowest ionization energy a Nitrogen b Fluroine c Sulphur d Oxygen.
The energy associated with the transition between two quantum states can be determined by 4En 2179x10-186- What is the energy of a mole of photon. The lower the first ionization energy. Which of the following has lowest ionization energy p3 CL.
From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon. Which of the following has lowest ionization energy. Therefore option C sulphur is correct.
Hence sodium electronic configuration 1 s 2 2 s 2 2 p 6 3 s 1 has the. What is 1st ionization energy. Energy released when excited electrons return to lower energy levels produce.
Its most stable. The attraction of the nucleus for the electron becomes less and it becomes easier to pull it away. Next Post Next What are neuropsychological tests used to assess.
For chemistry students and teachers. Beryllium has the lowest ionization energy. Cesium has the lowest ionization energy.
All of the above b. A 3026x10-19 kJmol c 1822 kJmol b 1822x105 kJmol d 5026 kJmol. The correct option is 1 P 3 which is due to high negative charge on phosphorus atom which will repel the electron and due to which very less energy is needed to remove an electron.
Which of the following has lowest ionisation energy. Which of the following elements has the lowest ionization energya. Ionization energies e none of the above C.
In the Periodic Table the minimum ionisation energy is found at alkali metals. This requires high amount of energy. From this trend Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy with the exception of Helium and Neon.
Which type of semiconductor is created by doping with atoms that contain more valence electron than the semiconductor material. Which of the following elements would have the lowest first ionization energy. Also the electronic configuration of sulphur is N e 3 s 2 3 p 4.
This is because the electron to be removed from the outer energy level is increasingly distant from the nucleus as a result of the atoms getting bigger. Which of the following is the correct designation for the orbital. By losing one electron sulphur can attain the half-filled state and hence achieve greater stability.
From the given elements Rb or Rubidium has the lowest ionization energy as it has lowest shielding effect so it easy to remove electron from its shell. Potassium option C has the smallest first ionization energy from the given elements. By losing one electron sulphur can attain the half filled state and hence achieve greater stability.
A Ba B Ca C Mg D Sr. Consider the given options. Asked 11 minutes 30 seconds ago3282022 34405 PM.
It is extremely radioactive. S kJmol associated with the transition from the n 2 energy level to the n 3 energy level. Check Answer and Solution for above question f.
Thus helium has the largest first ionization energy while francium has one of the lowest. Which of the following has the lowest ionization energy. What group has the lowest ionization energy.
If the ionization energy is low that means that it takes only a small amount of energy to remove the outermost electron. The element with the lowest ionization energy is cesium Cs. Log in for more information.
Nitrogen Fluorine Oxygen Sulphur So sulphur has the lowest ionization potential among all four. The first ionisation energy decreases on going down a group. Which of the following elements has the lowest ionization energy.

Periodic Trends In Ionization Energy Ck 12 Foundation
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic
How To Arrange The Following In The Order Of Decreasing Ionization Energy Li Na C O And F Quora

Inorganic Chemistry How Can I Relate The Reactivity Series To Electronegativity And Ionization Energy Chemistry Stack Exchange
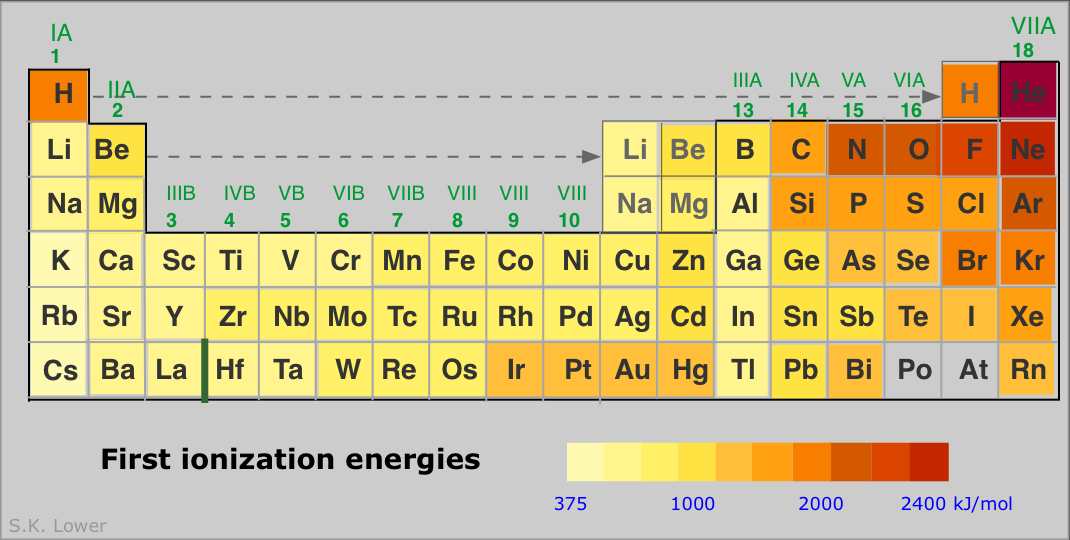
2 10 Periodic Properties Of The Elements Chemistry Libretexts
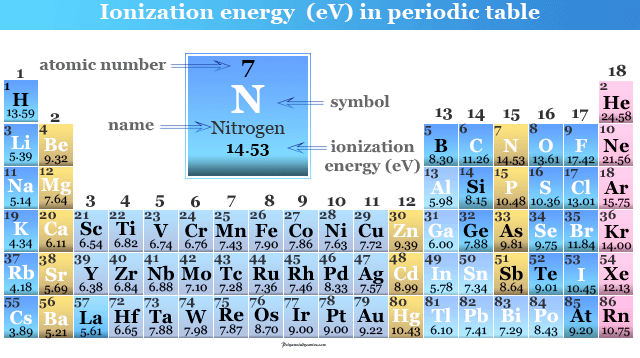
Ionization Energy Definition Equation Periodic Table Trends
Ionization Energy And Electron Affinity

What Group Of Elements Generally Have The Lowest Second Ionization Energy
What Is The Ionization Energy Of P Block Element Quora

Which One Has The Lowest First Ionization Energy Ca P Or Ci Quora

Ionization Energy Definition Chart Periodic Table Trend
8 2 Periodic Trends Physical Science

Ionization Energy Definition Chart Periodic Table Trend
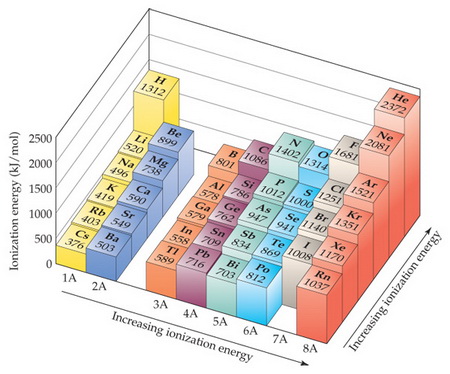
How Would You Arrange The Following Elements In Order Of Increasing Ionization Energy Te Pb Cl S Sn Socratic
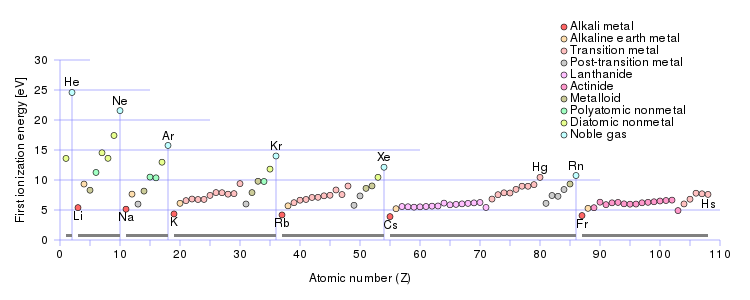
What Family Has The Lowest Ionization Energy Socratic

The Parts Of The Periodic Table


Comments
Post a Comment